| Preliminary Clinical Study of Anti-HBV-DC Combine Thymosin-a1 Treating the Inactive HBsAg Carrier |
发布时间: 2010-04-02 人气指数:3863 |
The 20th Conference of the Asian Pacific Association for the Study of the Liver
Hepatology International.2010, 4(1):157
Bang-Fu Wu1,2, Wen-Bao Zhu1, Jiang-Ying Yang2, Fang-Qin Li1, Yun Zhou2, Fu-Xin Lin1, Yan-Ping Fu1, Chun-Qiong Hou1, Hui-Hua Zhou1, Wei Zheng1, Wei Chen1, Jun Yang2, Xue-Song Li1
2 Guangzhou Pubang Biological Immune Technology Research Center, Room 904, D District, Guangzhou International Business Incubator, Guangzhou Science City, Luogang District, Guangzhou, China
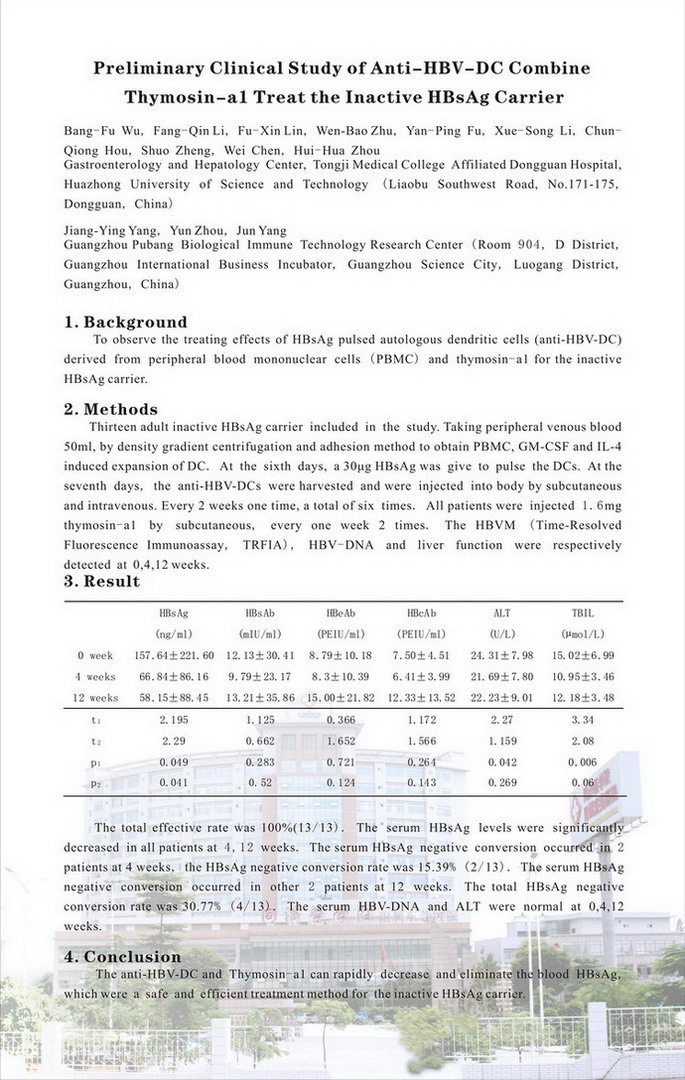
Background: To observe the treating effects of HBsAg pulsed autologous dendritic cells (anti-HBV-DC) derived from peripheral blood mononuclear cells (PBMC) combine thymosin-a1 for the inactive HBsAg carrier.
抗HBV-DC联合胸腺肽a1治疗非活动性HBsAg携带者的初步临床研究 第20届亚太肝脏研究会年会(北京) 海报展示(PP231) Hepatology International.2010, 4(1):157 华中科技大学同济医学院附属东莞医院消化肝病中心 吴邦富 李芳琴 凌佛鑫 朱文宝 付彦平 李雪松 侯春琼 郑硕 陈伟 周慧华 广州普邦生物免疫技术研究院 杨江英 周赟 杨军
目的 观察HBsAg致敏自体外周血单个核细胞(PBMC)来源的树突状细胞(抗HBV-DC)联合胸腺肽a1治疗非活动性HBsAg携带者的临床效果。 方法 非活动性HBsAg携带者13人接受临床研究。男9人,女4人,中位年龄27岁(18-43岁)。取肝素抗凝外周静脉血50ml,以密度梯度离心及贴壁法获得PBMC,GM-CSF和IL-4诱导扩增DC,第6天给予30μg的HBsAg致敏DC,第7天收获抗HBV-DC,皮下和静脉各注射1/2。每2周1次,共6次。皮下注射胸腺肽a1每次1.6mg,每周2次。分别于0、4、12周检测HBVM定量(时间分辨荧光免疫分析技术,TRFIA)、HBV-DNA定量及肝功能。 结果 0、4、12周的HBsAg分别为(157.64±221.60)ng/ml、(66.84±86.16)ng/ml(t=2.20,P=0.049)和(58.15±88.45)ng/ml(t=2.29,P=0.041)。全部患者治疗后4、12周的HBsAg均明显下降,其中2例4周时HBsAg转阴,转阴率15.39%(2/13),另2例12周时HBsAg转阴,总的HBsAg转阴率为30.77%(4/13)。治疗前后的ALT和HBV-DNA定量均正常。总有效率100%(13/13)。 结论 抗HBV-DC联合胸腺肽a1治疗,可快速降低非活动性HBsAg携带者血中HBsAg,部分患者的HBsAg得到清除,是一种安全、有效的治疗非活动性HBsAg携带者的方法。 |